Inventive step was approved based on the state of the art that there were still many unexplained issues in this matter on the priority date.
IP Court Case Summary:H22 (gyoke) 10203
May 28, 2012, the Intellectual Property High Court (IP High Court) reversed JPO board of appeal’s decision on the trial against examiner’s final rejection.
The title of the invention at issue is “Methods and compositions for inducing tumor-specific cytotoxicity”. Claim-1 is “A vector for expressing a sequence in a tumor cell, the vector comprising a polynucleotide comprising an H19 regulatory sequence operably linked to a heterologous sequence encoding a cytotoxic gene product, wherein the tumor cell is bladder carcinoma cell or bladder carcinoma”.
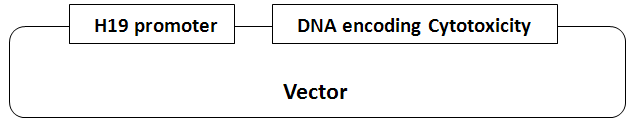
JPO board of appeal denied the inventive step of claim-1 because a person ordinarily skilled in the art could easily invent claimed invention (Claim-1) by the combination of citation 1 and citation 3-6.
The present invention has a feature for a combination of H19 promoter and a gene encoding a gene product of cytotoxicity. In contrast, the vector which combined DNA encoding a gene product of cytotoxicity with another promoter was described in citation 1. Also, citation 3 mentioned that H19 gene is actually expressed in a tumor cell, and citation 4-6 described the H19 promoter.
IP High Court mentioned as follows.
On the priority date, there were various kinds of trials to damage a tumor (cancer) by the delivery of a foreign gene, but they did not fully succeed because the expression of a foreign gene is difficult. Also, they did not because the activity of a promoter is not enough, and the immunoreaction of the host makes the expression difficulty. These facts were common recognition for a person those ordinarily skilled in the art, and the fact that an introduced gene is not sometimes expressed was known to those ordinarily skilled in the art. Also, according to citation 3, the possibility that endogenous H19 gene can be expressed in a bladder tumor cell is shown, and this gene must be a clue to functionalize a promoter and an enhancer in a tumor cell. However, as for the expected results to damage the tumor, there were still many unexplained issues in this matter on the priority date.
IP High Court finally reversed JPO board of appeal’s decision, and the inventive step was approved based on the state of the art that there were still many unexplained issues in this matter on the priority date.
<Comments>
Inventive step is not usually approved for the combination of the well-known promoter and the well-known structural gene. However, inventive step may be approved for even such an invention by insisting on “the state of the art at the time of the application”.
In this case, the inventive step was approved by showing “the state of the art at the time of the application” precisely. This precedent suggested the importance and the procedure of this approach.
http://www.ip.courts.go.jp/hanrei/pdf/20120530142940.pdf