Inventive step was approved in medicinal invention related to a specific dosage and administration, and JPO board of appeal’s decision was reversed.
IP Court Case Summary:2014(Gyo-Ke) 10045:
<Inventions>
The title of the invention at issue is “Use of Zoledronate for the Manufacture of a Medicament for the Treatment of Bone Metabolism Diseases”. Claim-1 is “the measures agent which includes 2-(imidazoI-1 yl)-1 -hydroxyethane-1 , 1 -diphosphonic acid (zoledronic acid) or the pharmaceutically acceptable salt as an active ingredient, in which 4 mg of zoledronic acid is administered in vein of the patient in need of bisphosphonate measures for 15 minutes”.
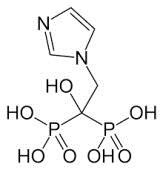
Citation-1 disclosed the measures agent which includes zoledronic acid as an active ingredient, in which 4 mg of zoledronic acid is administered in vein of the patient for 5 minutes.
<Comparison>
The identical features between claimed invention (Claim-1) and cited invention (Citation-1) are “the measures agent which includes 2-(imidazoI-1 yl)-1 -hydroxyethane-1 , 1 -diphosphonic acid (zoledronic acid) or the pharmaceutically acceptable salt as an active ingredient, in which 4 mg of zoledronic acid is administered in vein of the patient in need of bisphosphonate measures for the specific term”. The difference between those two is the term of vein administration that is “15 minutes” (Claim-1) and “5 minutes” (Citation-1).
<JPO Board of Appeal>
JPO board of appeal denied the inventive step of claim-1 because a person ordinarily skilled in the art could easily reach “15 minutes” from “5 minutes”. The reasons are as follows;
– Citation-1 is related to phase II (clinical test), which means some side effects might be found in phase III. In order to solve the problem, a person skilled in the art could easily decide dosage and administration of zoledronic acid.
– Citation-3 disclosed that slow administration of bisphosphonate is preferable because rapid administration caused renal insufficiency.
<IP High Court>
IP High Court finally reversed JPO board of appeal’s decision, and the inventive step was approved. The reasons are as follows;
– According to phase II in Citation-1, 5 minute administration is enough safe and there was no description about the problem on safety. Then, there was no incentive to try longer time than 5 minutes.
– Citation-3 is related to bisphosphonate in 1st generation, and Claim-1 is related to bisphosphonate in 3rd generation. The description in Citation-3 cannot be directly applied to bisphosphonate in 3rd generation.
<Comments>
Medicinal invention related to a specific dosage and administration is examined in the JPO according to Examination Guideline. In Chapter 3 (Medicinal Inventions) in the Part VII of Examination Guideline, the optimization of dosage and administration of a medicine is among exercise of ordinary creativity of a person skilled in the art. In this case, the decision by JPO Board of appeal looks under this part.
<Note>
Medicine characterized in the medicinal use of an application to a specific disease with a specific dosage and administration is explained in Examination Guideline (Part VII, Chapter 3) as follows; “As for a specific disease, in order to solve a problem well known to a person skilled in the art such as the increase of a medicinal effect, the reduction of an adverse effect or the improvement in drug compliance, the optimization of dosage and administration of a medicine is among exercise of ordinary creativity of a person skilled in the art. Accordingly, in the case where the advantageous effect compared with the cited invention can be foreseen by a person skilled in the art, the inventive step is usually denied, even if the claimed medicinal invention is novel compared with the cited invention in that applied disease does not differ but dosage and administration differs from each other. (revised in October of 2009)”
http://www.ip.courts.go.jp/app/files/hanrei_jp/754/084754_hanrei.pdf