IP High Court Case Summary: 2020 (Gyo-ke) 10098
INTELLECTUAL PROPERTY HIGH COURT PARTIALLY REVOKES BOARD OF APPEAL DECISION THAT INVALIDATED REGISTRATION OF PATENT TERM EXTENSION: “ANTIPRURITIC AGENT CASE”
1. Outline of this Case
This is the case of the revocation of the JPO Board of Appeal’s decision (Invalidation Trial No. 2020-800004) that invalidated the registration of extension of the duration of the patent (JP Patent No. 3531170) concerning the invention entitled “Antipruritic Agent”. The points at issue are as follows:
(i) Defendant eligibility of the intervenor of the JPO’s Trial Procedure
(ii) Necessity to receive the disposition (the present disposition) under Pharmaceuticals and Medical Devices Act Article 14 in order to work this patented invention
(iii) Possibility to invalidate only a part of the registration of extension in case there is a reason for an invalidation in a part of the registration of extension
The Intellectual Property High Court (IPHC) accepted the defendant eligibility, and decided that it was necessary to receive the present disposition in order to work this patented invention and that it is possible to invalidate only a part of the registration of extension. Then, the IPHC revoked a part of the JPO Board of Appeal’s decision.
2. Present Invention
The invention described in claim 1 (the present invention 1) is as follows: (total number of claims: 36)
<Claim 1>
An antipruritic agent comprising an opiate κ receptor agonist as an active component, wherein the opiate κ receptor agonist is represented by the general formula (I):
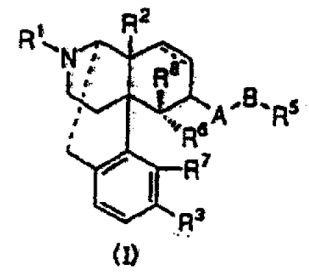
[in the formula (I), ・・・]
3. Present Disposition
This application for the registration of patent term extension (2017-700310) is based on the approval to manufacture and sell the medicine of “REMITCH OD tablet 2.5μg” (brand name). The present disposition is as follows:
<Present Disposition>
a. Requested term of extension: 5 years
b. Disposition as a reason for the registration of extension: Approval of the medicine prescribed in Pharmaceuticals and Medical Devices Act Article 14(9)
c. Identification number of the disposition: 22900AMX00538000
d. Date of the disposition: October 22, 2017
e. Medicine that became subject to the disposition: REMITCH OD tablet 2.5μg (brand name) (active component: Nalfurafine hydrochloride)
f. Specified use of the medicine that became subject to the disposition
(Before amendment)
Improvement of Pruritus in the following patients:
(only when an effect is insufficient by existing treatment)
a dialysis patient, a patient with chronic liver disease
(After amendment)
Improvement of Pruritus in the following patients:
(only when an effect is insufficient by existing treatment)
a dialysis patient (excepting a hemodialysis patient), a patient with chronic liver disease
4. JPO Examiner’s Examination
Before this disposition, the prior disposition for this medicine was approved on March 30, 2017 as follows:
<Prior Disposition>
Efficacy and Effect
Improvement of Pruritus in the following patients:
(only when an effect is insufficient by existing treatment)
a hemodialysis patient, a patient with chronic liver disease
During the JPO examiner’s examination of this application for the registration of patent term extension, the reason for refusal was notified due to the fact that “a hemodialysis patient” was included in “specified use of the medicine that became subject to the disposition” in the prior disposition. Therefore, “specified use of the medicine that became subject to the disposition” was amended to “a dialysis patient (excepting a hemodialysis patient), a patient with chronic liver disease”, and the extension of five years was registered on July 25, 2018.
5. JPO Board of Appeal’s Decision
The defendant (Sawai Pharmaceutical) filed an invalidation trial (Invalidation Trial No 2020-800004) for the registration of patent term extension by the plaintiff (Toray) on January 23, 2020. The defendant (Nipro) requested an intervention under Patent Act Article 148(1) on April 10, 2020, and the JPO Board of Appeal decided to accept the intervention on June 11 of the same year. The JPO Board of Appeal’s Decision is as follows:
(1) Determination of active component of the present medicine
The active component of the present medicine is Nalfurafine hydrochloride according to the description of the active component of the present medicine, “a pharmaceutical interview form” and “package insert information”.
“An antipruritic agent” of the present invention includes the chemical compound of the general formula (I) as an active component which is interpreted to mean an “unsalted” compound according to the description of this patent and examination procedure.
Therefore, the present medicine does not correspond to the present invention. Then, it is not necessary to receive the disposition (the present disposition) under the Pharmaceuticals and Medical Devices Act in order to work this patented invention.
(2) Partial invalidation of specified use in case of the registration of extension
It was not necessary to receive the disposition (the present disposition) under the Pharmaceuticals and Medical Devices Act in order to work this patented invention for a hemodialysis patient because the present invention could already be worked in case of the treatment for a hemodialysis patient.
Therefore, “Improvement of Pruritus in the following patients (only when an effect is insufficient by existing treatment)” has a reason for rejection.
(3) Conclusion
As above, this registration of patent term extension should be invalid.
6. Decision by IPHC
The IPHC accepted the defendant eligibility, and decided that it was necessary to receive the present disposition in order to work this patented invention and that it is possible to invalidate only a part of the registration of extension. Then, the IPHC revoked a part of the JPO Board of Appeal’s decisions.
(1) Defendant eligibility of the intervener of the JPO’s Trial Procedure
Patent Act Article 148(1) clearly states that an intervener under Patent Act Article 148(1) can intervene in the invalidation trial for a patent or the invalidation trial for the registration of patent term extension by stipulating that “a person who may file a request for a trial under Patent Act Article 132(1) may intervene in the trial as a demandant until the conclusion of the proceedings.” Therefore, the intervenor under Patent Act Article 148(1) can be interpreted to have defendant eligibility as an intervenor under Patent Act Article 179(1).
Therefore, the IPHC decided that the defendant (Nipro) has defendant eligibility as interlocutory judgment on December 2, 2020 (2020 (Gyo-ke) 10098).
(2) Determination of active component of the present medicine
From the viewpoint of the purpose of the registration of patent term extension, the active component of the present medicine should be determined substantially, but not formally from the description of the active component in written approval under the Pharmaceuticals and Medical Devices Act Article 14. This interpretation can follow the Supreme Court Decision 2014 (Gyo-hi) 356 (November 17, 2015) “Avastin Case”.
The active component of the present medicine should not be decided as “Nalfurafine hydrochloride” according to the description of the active component of the present medicine, “a pharmaceutical interview form” and “package insert information”. However, an active component which makes the efficacy and effect should be not only “Nalfurafine hydrochloride” but also “free Nalfurafine” in consideration of “significance of a form of the salt in pharmaceutical compound”, “recognition of those skilled in the art” and “contents of clinical tests for approval to manufacture and sell the medicine”.
Therefore, the JPO Board of Appeal’s decision, stating that “the active component of the present medicine is only Nalfurafine hydrochloride”, “Nalfurafine is not an active component of the present medicine”, and “it is not necessary to receive the disposition (the present disposition) under the Pharmaceuticals and Medical Devices Act in order to work this patented invention”, has errors.
(3) Partial invalidation of specified use in case of the registration of extension
Clearly, it is not necessary to receive the present disposition under the Pharmaceuticals and Medical Devices Act in order to work this patented invention for a patient with chronic liver disease because the present invention could be already worked in case of the treatment for a patient with chronic liver disease by the prior disposition. Therefore, “a patient with chronic liver disease” in the present registration of extension has a reason for rejection.
In case there is a reason for invalidation of a part of the registration of extension, there is no reason that the registration of extension should be totally invalidated. In case it is not necessary to receive the disposition under Patent Act Article 67(2) (presently, Article 67(4)) in order to work a part of the registration of extension, only the part can be invalidated in the invalidation trial).
The IPHC confirmed the JPO Board of Appeal’s decision concerning partial invalidation of specified use in case of the registration of extension because the present invention could be already worked in case of the treatment for a patient with chronic liver disease by the prior disposition.
7. Comments
(1) Defendant eligibility of an intervenor of the JPO’s Trial Procedure
The IPHC decided that the intervenor of an invalidation of the registration of extension in a trial procedure has defendant eligibility in the case of the lawsuit against a trial decision.
Patent Act Article 179(1) stipulates the defendant eligibility in the trial for invalidation of a patent and the retrial as well as the trial for invalidation of the registration of extension.
Patent Act Article 148(1) states that the intervenor can intervene as a demandant not only in the trial for invalidation of the registration of extension but also in the trial for invalidation of a patent in the trial procedure, and Patent Act Article 171(1) states that an intervenor may file a request for a retrial against a final and binding trial decision.
Therefore, defendant eligibility of the intervenor of the JPO’s trial procedure can also be applied to the lawsuit against the trial decision of the invalidation for a patent and the retrial decision as well as the trial decision of the invalidation for the registration of extension. Going forward, it is important to consider the intervenor in trial procedure in consideration of the following lawsuit against the trial decision.
(2) Determining active component of the present medicine
The IPHC decided that the active component of the present medicine should be determined substantially, but not formally from the description of the active component in written approval under the Pharmaceuticals and Medical Devices Act Article 14.
According to “JPO Examination Guideline” (part IX, 2.4), in a request of the application for the registration of extension, “a product that became the subject of the disposition” and “in a case where a particular use in which the product is used is defined in the disposition, said particular use” shall be described, and “in the case of a pharmaceutical product, the efficacy and effect stated in the written approval” shall be described.
In addition, according to “JPO Examination Guideline” (part IX, 3.1.1), in examination of the application for the registration of extension, “when an act of manufacturing and distribution of drug products or an act of manufacturing and import of agricultural chemicals subject to the present disposition does not fall under an act of working of the patented invention pertaining to an application for registration of extension for pharmaceutical inventions,” the examiner should notify the reason for refusal. However, how to make a substantial decision from the statement of written approval is not shown in “JPO Examination Guideline”.
Going forward, it is important to monitor the trend of examination of the application for the registration of extension in order to understand how to make a substantial decision from the statement of written approval. Note that the accumulation of further precedents is necessary to clarify the examination of extension, and future court cases should be carefully monitored.
(3) Partial invalidation of specified use in case of the registration of extension
The IPHC decided that when it is not necessary to receive the disposition to work a part of the use registration of extension, only the part can be invalidated in the invalidation trial for the registration of extension. Specifically, it was decided that in case there is a reason for invalidation of a part of the registration of extension, there is no reason that the registration of extension should be totally, not partially, invalidated.
Going forward, it is important to consider the fulfillment of the requirements for the registration of extension while considering “respective uses” in case multiple uses were approved in the disposition.
8. Related cases
Concerning the present patent (JP Patent No.3531170), the IPHC decided four lawsuits against trial decision concerning the registration of extension on the same day (March 25, 2021). All four trial decisions were revoked by the IPHC.
(1) IPHC 2020 (Gyo-ke) 10063
Lawsuit against trial decision against examiner’s decision of refusal (Trial against examiner’s final rejection No. 2018-7539) concerning the application for registration of extension of duration of the patent (2017-700154) based on the following disposition under Pharmaceuticals and Medical Devices Act Article 14
a. Medicine that became subject to the disposition: REMITCH OD tablet 2.5μg (brand name) (active component: Nalfurafine hydrochloride)
b. Specified use of the medicine that became subject to the disposition
Improvement of Pruritus in the following patients:
(only when an effect is insufficient by existing treatment)
a dialysis patient, a patient with chronic liver disease
(2) IPHC 2020 (Gyo-ke) 10096
Lawsuit against trial decision of invalidation of registration of extension of duration of the patent (Invalidation Trial No. 2020-800002) concerning the application for the registration of extension (2015-700061) based on the following disposition under Pharmaceuticals and Medical Devices Act Article 14
a. Medicine that became subject to the disposition: NOPICOL capsule 2.5μg (brand name) (active component: Nalfurafine hydrochloride)
b. Specified use of the medicine that became subject to the disposition
Improvement of Pruritus in a patient with chronic liver disease
(only when an effect is insufficient by existing treatment)
(3) IPHC 2020 (Gyo-ke) 10097
Lawsuit against trial decision of invalidation of registration of extension of duration of the patent (Invalidation Trial No. 2020-800003) concerning the application for the registration of extension (2017-700309) based on the following disposition under Pharmaceuticals and Medical Devices Act Article 14
a. Medicine that became subject to the disposition: REMITCH capsule 2.5μg (brand name) (active component: Nalfurafine hydrochloride)
b. Specified use of the medicine that became subject to the disposition
Improvement of Pruritus in the following patients:
(only when an effect is insufficient by existing treatment)
a dialysis patient (excepting a dialytic treatment patient), a patient with a chronic liver disease
(4) IPHC 2020 (Gyo-ke) 10098 (*This case explained above)
Lawsuit against trial decision of invalidation of registration of extension of duration of the patent (Invalidation Trial No. 2020-800004) concerning the application for the registration of extension (2017-700310) based on the following disposition under Pharmaceuticals and Medical Devices Act Article 14
a. Medicine that became subject to the disposition: REMITCHOD 2.5μg (brand name) (active component: Nalfurafine hydrochloride)
b. Specified use of the medicine that became subject to the disposition
Improvement of Pruritus in the following patients:
(only when an effect is insufficient by existing treatment)
a dialysis patient (excepting a dialytic treatment patient), a patient with chronic liver disease
https://www.ip.courts.go.jp/app/files/hanrei_jp/246/090246_hanrei.pdf
<Related Provisions>
[Patent Act]
Article 67 (Duration of Patent Right)
(4) Where there is a period during which the patented invention is unable to be worked because approvals prescribed by relevant Acts that are intended to ensure the safely, etc. or any other disposition designated by Cabinet Order as requiring considerable time for the proper execution of the disposition in light of the purpose, procedures, etc., of such a disposition need to be obtained for the working of the patented invention, the duration of the patent right may be extended, upon the filing of a request for the registration of extension of the duration, by a period not exceeding 5 years.
Article 67-7 (Registration of extension of duration)
(1) Where an application for the registration of extension of the duration of a patent right falls under any of the following items, the examiner shall render the examiner’s decision to the effect that the application is to be refused:
(i) where the disposition designated by Cabinet Order under Article 67(4) is not deemed to have been necessary to obtain for the working of the patented invention;
Article 132 (Joint Trial)
(1) Where two or more persons file a request for a trial for patent invalidation or a trial for invalidation of the registration of extension of duration concerning the same patent right, the request may be filed jointly.
Article 148 (Intervention)
(1) A person who may file a request for a trial under Article 132(1) may intervene in the trial as a demandant until the conclusion of the proceedings.
(2) An intervenor under the preceding paragraph may continue the trial procedures even after the withdrawal of the request for a trial by the original party.
(3) A person with an interest in the result of the trial may intervene in the trial to assist one of the original parties until the conclusion of the proceedings.
(4) The intervenor under the preceding paragraph may undertake all trial procedures.
(5) Where there is a ground for interruption or suspension of trial procedures on behalf of the intervenor under paragraph (1) or (3), the said interruption or suspension shall have effect on the original parties.
Article 171 (Request for Retrial)
(1) A party or an intervenor may file a request for a retrial against a final and binding trial decision.
Article 179 (Appropriate party as defendant)
In an action under Article 178(1), the Commissioner of the Patent Office shall be the defendant; provided, however, that in the case of an action against a trial decision in a trial for patent invalidation, or a trial for invalidation of the registration of extension of duration, or in a retrial under Article 171(1) against a final and binding trial decision in such trial, the demandant or the demandee in the trial or retrial shall be the defendant.
[Pharmaceuticals and Medical Devices Act]
Article 14 (Marketing Approval for Pharmaceuticals, Quasi-Pharmaceutical Products and Cosmetics)
(1) A person who intends to market pharmaceuticals (excluding pharmaceuticals with specified standards designated by the Minister of Health, Labour and Welfare), quasi-pharmaceutical products (excluding quasi-pharmaceutical products with specified standards designated by the Minister of Health, Labour and Welfare) or cosmetics which contain components specified by the Minister of Health, Labour and Welfare must obtain approval from the Minister of Health, Labour and Welfare for each such item.
(9) When a person who has received approval prescribed in paragraph (1) wishes to make a minor change to approved items (excluding cases where such change is a minor change specified by Order of the Ministry of Health, Labour and Welfare), they must receive approval for such minor change from the Minister of Health, Labour and Welfare. In this case the provisions of paragraph (2) through the preceding paragraph apply mutatis mutandis.