Tokyo District Court Judgement of May 15, 2025 (2023 (Wa) 70527 and 2024 (Wa) 70016)
THE “SPRYCEL CASE” IN WHICH INFRINGEMENT OF A PHARMACEUTICAL PATENT WITH AN EXTENDED TERM WAS DENIED
1. Case Overview
(1) Main Lawsuit
In this main lawsuit, Plaintiff, who manufactures and sells the formulations (Plaintiff’s Products) listed in the attached Schedule, asserted against Defendant, the patent holder of Patent No. 3989175 (hereinafter referred to as the “Patent” and the patent right thereof as the “patent right”) for an invention entitled “Cyclic Protein Tyrosine Kinase Inhibitor”, that the effect of the Patent, which had been granted a term extension, does not extend to Plaintiff’s production, assignment, or offer to assign the Plaintiff’s Products. The main lawsuit was dismissed for lack of declaratory interest, and the focus of the substantive proceedings shifted to the counterclaim.
(2) Counterclaim
In this counterclaim, Defendant alleged that the Plaintiff’s Products fall within the technical scope of the invention described in claim 9 of the patent (the “Invention”), and that the patent right after extension extends to Plaintiff’s production of the Plaintiff’s Products. Defendant then sought compensation for damages of 100 million yen from Plaintiff based on the tort of infringement of the patent right (Article 709 of the Civil Code; Article 102, Paragraphs 1-3 of the Patent Act, regarding damages).
2. The Patent and Its Extension
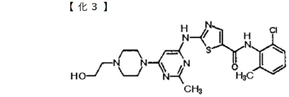
Claim 9 of the claims of the patent is as follows:
[Claim 9] A compound of the following formula or a salt thereof.
Defendant applied for and obtained an extension of the patent term. The extension expired on January 27, 2024.
3. Plaintiff’s Actions
On October 4, 2023, Plaintiff obtained approval for efficacy, effects, dosage and administration for its products as generic versions of “Sprycel Tablets 20 mg” and “Sprycel Tablets 50 mg” (collectively referred to as “Sprycel Tablets”), which are manufactured and sold by Defendant as formulations of dasatinib.
In conjunction with obtaining the additional approval, Plaintiff amended the contents of the package inserts for Plaintiff’s Products and began manufacturing and selling Plaintiff’s Products to include the use of chronic myeloid leukemia specified by the additional efficacy, effects, dosage and administration provided by the approval. There is no dispute between the parties that Plaintiff’s Products contain a compound within the technical scope of the Invention.
<Item List>
1. Brand Name: Dasatinib Tablets 20mg (SAWAI)
Efficacy-effect:
1. Chronic myeloid leukemia
2. Relapsed or refractory Philadelphia chromosome-positive acute lymphoblastic leukemia
2. Brand Name: Dasatinib Tablets 50mg (SAWAI)
Efficacy-effect:
1. Chronic myeloid leukemia
2. Relapsed or refractory Philadelphia chromosome-positive acute lymphoblastic leukemia
(Chronic Myeloid Leukemia)
(1) Chronic Phase
The usual adult dosage is 100 mg of dasatinib administered orally once daily.
The dose may be increased up to 140 mg once daily, depending on the patient’s condition.
(2) Transitional or Acute Phase
The usual adult dosage is 70 mg of dasatinib administered orally twice daily.
The dose may be increased up to 90 mg twice daily, depending on the patient’s condition.
(Relapsed or Refractory Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia)
The usual adult dosage is 70 mg of dasatinib administered orally twice daily.
The dose may be increased up to 90 mg twice daily, depending on the patient’s condition.
4. Issues
The claims in this lawsuit, both the primary and alternative claims, are unlawful and lack a basis for confirmation. As a result, the issues (counterclaims) are as follows. Issue 1 is discussed below.
(1) The validity of the patent right extensions and Plaintiff’s Products (Issue 1)
(2) Whether there are grounds for invalidating the extension registrations
in this case (Issue 2)
A. The acts whose prohibition was lifted by the dispositions at issue do not fall within the scope of acts constituting the practice of the Invention at issue
(Issue 2-1)
B. The extension periods were excessive (Issue 2-2)
(3) Defendant’s damages (Issue 3)
5. Court Decision
(1) Scope of the Extended Patent Right (Patent Act Article 68-2)
The court stated that “The effect of a patented invention related to an extended patent right extends not only to the ‘product’ (pharmaceutical) specified by the ‘ingredients, quantity, usage, dosage, efficacy, and effects’ prescribed in the disposition, but also to products substantially identical to such products as pharmaceuticals, and third parties should expect this. Therefore, even if there are differences between the subject product and the product specified in the disposition, if such differences are only minor or merely superficial, the subject product is considered to be included in the products substantially identical to the subject product as pharmaceuticals and therefore falls within the scope of the extended patent right. …Whether a difference is minor or merely superficial should be determined based on the content of the patented invention, in relation to that content, by comparing and examining the similarity of the technical features and effects of the subject product and the ‘product’ specified by the ‘ingredients, quantity, usage, dosage, efficacy, and effects’ prescribed in the disposition, and taking into account the technical common knowledge of a person skilled in the art.”
The court then ruled that “one example of a product that falls within the category of something that is substantially identical as a pharmaceutical to the ‘product’ specified by the ‘ingredients, quantity, usage, dosage, efficacy, and effects’ specified in the Cabinet Order is when, in a patented invention for which an extension of the patent term has been granted for an invention that features only the active ingredients of a pharmaceutical, the product in question adds or converts some of the non-active ‘ingredients’ based on well-known or commonly used technology at the time of filing the application for the Cabinet Order.
In this case, the difference falls under the category of “slight differences or differences that are merely formal when viewed as a whole,” and the product in question should be considered to be something that is substantially identical as a pharmaceutical to the product that was the subject of the Cabinet Order.”
(2) Consideration of this Case
The court ruled that, “The invention at issue is directed solely to a novel compound that serves as an active pharmaceutical ingredient and is understood to be a patented invention characterized solely by the active pharmaceutical ingredient. As noted above, in a patented invention for which an extension of the patent term has been granted for a patented invention characterized solely by the active pharmaceutical ingredient, if the subject product has added or converted a portion of an ingredient that is not an active ingredient based on well-known or commonly used technology at the time of filing the application for the Cabinet Order, the subject product should be considered to be substantially identical to the product that was the subject of the Cabinet Order as a pharmaceutical.”
The court then compared Plaintiff’s formulation with Sprycel tablets, and with regard to the non-active “ingredients,” Sprycel tablets add PEG 400 as an additive, while Plaintiff’s Products do not, and instead add carnauba wax as a coating agent. After examining the differences in the hygroscopicity and stability of the formulation, the court ruled that “…the addition or conversion of the above additives appears to have been made in light of issues arising from differences in the active ingredients between Sprycel tablets (dasatinib hydrate) and Plaintiff’s Products (dasatinib anhydrate). There is no adequate evidence to prove that the addition or conversion of such additives is based on well-known or commonly used technology. Rather, it appears that Plaintiff added or converted these additives based on its own technology, etc., to bring the dissolution behavior of its product closer to that of Sprycel tablets, or to achieve bioequivalence with Sprycel tablets. Therefore, Plaintiff’s Products cannot be said to be substantially identical to Sprycel tablets as a pharmaceutical product.”
6. Comments
This judgment held that the basic concept of the effect of a patent right whose term has been extended (Patent Act Article 68-2) is that it applies not only to “products” (pharmaceuticals) specified by the Cabinet Order based on their “ingredients, quantity, usage, dosage, efficacy, and effects,” but also to products that are substantially identical as pharmaceuticals. This concept was also held in the “Oxaliplatin Case” (Intellectual Property High Court (Grand Panel) Judgment of January 20, 2017).
Furthermore, with regard to the method of determining substantial identity, this judgment stated that “whether a difference is slight or merely formal, taken as a whole, should be determined based on the content of the patented invention, in relation to that content, by comparing and examining the identity of the technical features and effects of the “product” specified by the “ingredients, quantity, usage, dosage, efficacy, and effects” specified in the Cabinet Order with the subject product, taking into account the technical common knowledge of a person skilled in the art.” This concept was also held in the “Oxaliplatin Case.”
Furthermore, this judgment indicated that one type of product that falls under the category of substantially identical is “a case in which, in a patented invention for which an extension of the registration period has been granted for a patented invention characterized only by the active ingredient of a pharmaceutical, the target product has added or converted a part of an ingredient that is not the active ingredient based on well-known or commonly used technology at the time of filing the application for the Cabinet Order.” This type of case was also ruled in the “Oxaliplatin Case.”
However, this judgment concluded that cases in which the ingredient differs to the extent of the “additive” are not “substantially identical,” and therefore the extended patent right does not apply. Regarding this point, a thorough comparative examination is required from the perspective that “the sameness of the technical features and effects with the subject product should be compared and judged based on the technical common knowledge of a person skilled in the art.” However, this judgment concluded, prior to such a thorough comparative examination, that “there is no adequate evidence to recognize that the addition or conversion of the additive is based on well-known or commonly used art.” This has led to criticism that the judgment was concluded by merely applying the example type to a perfunctory application. (For example, “Formal Decision Shakes the Foundation of the Patent Extension System in the Sprycel Generic Litigation” (Pharmaceutical “Patent” Case Law Blog).)
In this regard, the Intellectual Property High Court Judgment of May 27, 2025, ruling in the “Nalfurafine Case” (2021(Ne)10037) held that in an infringement lawsuit over a patent whose term had been extended, cases in which the ingredients differed to the extent of the “additive” were deemed “substantially identical,” and the extended patent right still applied. In this case, comparative consideration was conducted to determine whether the additive was added in a way that did not exhibit a pharmacological effect at the dosage of the formulation and did not interfere with the therapeutic effect of the active ingredient.
Further case law accumulation is needed to increase the predictability of decisions regarding patent extensions, and we will be paying close attention to future case law developments.