IP High Court Judgment of May 27, 2025 (2021 (Ne) 10037)
THE “ANTIPRURITIC AGENT CASE” IN WHICH INFRINGEMENT OF A PHARMACEUTICAL PATENT WHOSE TERM HAD BEEN EXTENDED WAS UPHELD
1. Outline of this Case
In this case, the appellant (plaintiff: Toray), the patent holder of the patent (JP Patent No. 3531170) for an invention titled “Antipruritic Agent,” filed a lawsuit against the appellees (defendants: Sawai Pharmaceutical and Fuso Pharmaceutical Industries) alleging that the defendants’ manufacturing, sales, etc. of the defendant’s formulation (antipruritic agent) during the extended patent term constituted infringement of the patent, and sought damages from the defendants.
In the original trial (Tokyo District Court, 2018 (Wa) 38504 and 38508), the appellant’s (first-instance plaintiff) claim was dismissed on the grounds of “non-infringement” of the patent. Dissatisfied with this decision, the appellant appealed to the Intellectual Property High Court.
2. Summary of the Original Trial
In the original trial, the court held that the infringement claimed in the infringement claims (literal infringement and equivalent infringement) was not satisfied because the infringement claimed in the infringement claims contained “Nalfurafine (free form)” as an active ingredient, while the defendant’s formulation contained “Nalfurafine hydrochloride” as an active ingredient. Furthermore, in light of the prosecution history, the plaintiff deliberately excluded the use of “Pharmacologically acceptable acid addition salts” as an active ingredient from the patent claims, and therefore the doctrine of equivalents did not apply. The court dismissed both the plaintiff’s claims (for literal infringement and equivalent infringement). Dissatisfied with the original ruling, the plaintiff appealed to the Intellectual Property High Court.
3. Patented Invention
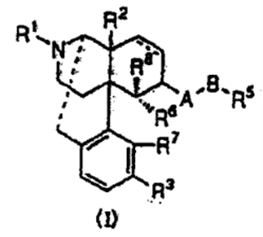
Claim 1 of the present patent recites “Nalfurafine (free form)” as follows and does not use the expression “acid addition salt.”
[Claim 1]
An antipruritic agent comprising, as an active ingredient, an opioid κ receptor agonist compound represented by the following general formula (I) [in the formula (I), ・・・].
4. Registration of patent term extension
An application for patent term extension was filed on June 29, 2017, and the extension was granted on August 11, 2021 (to November 26, 2024).
The subject of the pharmaceutical approval (disposition in question) that was the basis for the extension is as follows:
<This Disposition>
– Disposition number identifying the reason for the registration extension:
22900AMX00538000
– Medicine that became subject to the disposition: REMITCH OD tablet 2.5μg (brand name)
Active component: Nalfurafine hydrochloride
– Specified use of the medicine that became subject to the disposition
Improvement of Pruritus in the following patients:
(only when an effect by existing treatment is insufficient)
Hemodialysis patients, Chronic liver disease patients
Defendant Sawai Pharmaceutical filed a request for invalidation of the extension registration on September 29, 2021, but the Japan Patent Office ruled on February 28, 2024, that the request for invalidation was invalid. Defendant Sawai Pharmaceutical appealed to the Intellectual Property High Court seeking reversal of the decision, but the request was dismissed on May 27, 2025 (Intellectual Property High Court Judgment 2024 (Gyo-Ke) 10033).
5. Defendant Formulations
The defendants have been manufacturing, selling, etc., the following defendant formulations since they were listed in the National Health Insurance (NHI) drug price standards. The defendant formulations contain Nalfurafine hydrochloride as their active ingredient.
<List of Defendant Products>
– Sawai Pharmaceutical: Nalfurafine Hydrochloride OD Tablets 2.5µg “Sawai”
– Fuso Pharmaceutical Industries: Nalfurafine Hydrochloride OD Tablets 2.5µg “Fuso”
6. Intellectual Property High Court Decision
(1) Technical Scope of the Invention and the Defendant’s Formulation
In this decision, the court stated that “at the time of filing of the patent, it was recognized as common general technical knowledge that drugs should be formulated in the form of an acid addition salt to improve their solubility and stability,” and that “the application process does not indicate that antipruritic agents containing pharmacologically acceptable acid addition salts as active ingredients were intentionally excluded from claim 1.” The court further determined that the invention in question refers to an antipruritic agent in which a compound represented by general formula (I), whether in the form of an acid addition salt or not, is dissolved and absorbed in vivo and exerts its pharmacological action as an “active ingredient” based on its opioid κ receptor agonism.
The court also determined that the defendant’s formulation is a pharmaceutical product containing Nalfurafine in the form of its acid addition salt, Nalfurafine hydrochloride, which is dissolved and absorbed in vivo and exerts its antipruritic effect based on its opioid κ receptor agonism.
Based on the above, it was determined that the defendant’s formulation satisfies the constituent elements of the claims of the present invention (Claim 1) and falls within its technical scope.
(2) Scope of Patent Rights with Registered Extended Term
In this judgment, the court interpreted the effect of a patent right with registered extended term (Patent Act Article 68-2) as extending the effect of the extended patent right to products that are deemed to be “substantially identical” in terms of “ingredients, quantity, usage, dosage, efficacy, and effects” to the plaintiff’s formulation.
The court held that both the plaintiff’s and defendant’s formulations are antipruritic agents containing the active ingredient Nalfurafine, a κ receptor agonist compound represented by general formula (I), and that their “active ingredient, quantity,” “usage, dosage, efficacy, and effects,” as well as their dosage form (OD tablets), differ only in terms of the amount of additives excluding the active ingredient.
The court also held that, generally, additives are added to formulations that do not exhibit pharmacological effects at the dosage level and do not interfere with the therapeutic effects of the active ingredient. The court also held that the additives in this case were not deemed to have any technical significance different from this.
Based on the above, the judgment held that the differences in the additives between the plaintiff’s formulation and the defendant’s formulation were minor or merely formal differences overall, and that the defendant’s formulation was substantially identical to the plaintiff’s formulation, and therefore the effect of the patent right, the term of which had been extended, extended to cover the manufacture, sale, etc. of the defendant’s formulation.
7. Comments
(1) Technical Scope of the Invention and the Defendant’s Formulation
In this judgment, the invention was interpreted as referring to an antipruritic agent in which a compound represented by general formula (I), regardless of whether it is in the form of an acid addition salt, is dissolved and absorbed in the body and exerts its pharmacological action as an “active ingredient” based on its opioid κ receptor agonism.
In this judgment, even when the “free form” is recited in the claims but the “acid addition salt” is not, the patent right also extends to the “acid addition salt.” This judgment was based on the interpretation of the “active ingredient” that exerts its pharmacological action in the body. However, this interpretation must be applied after taking into consideration the description in the specification and drawings, common general knowledge, etc. In this case, it was shown that the drug was in the form of an acid addition salt to improve its solubility and stability. In future practice, when interpreting the term “acid addition salt,” careful consideration of its technical significance will be necessary.
As to whether this applies to salt forms other than “acid addition salts,” such as sodium salts and potassium salts, we will need to wait for the accumulation of case law, and it will be important to keep an eye on future developments in this area.
(2) Scope of Patent Rights with Registered Extended Term
The interpretation of the effect of patent rights with registered extended term (Patent Act Article 68-2) that the effect of an extended patent right extends to products that are deemed to be “substantially identical” to the plaintiff’s formulation in terms of “ingredients, quantity, usage, dosage, efficacy, and effects” was also held in the “Oxaliplatin Case” (Intellectual Property High Court (Grand Panel) Judgment of January 20, 2017).
This judgment recognized that the effect of an extended patent right extends to cases where the ingredients differed to the extent of “additives,” as they were deemed to be “substantially identical.” In this case, sufficient consideration was given to the technical significance of the additives, including that the additives must not exhibit any pharmacological effect at the dosage of the formulation and must not interfere with the therapeutic effects of the active ingredient.
On this point, the Tokyo District Court’s May 15, 2025 ruling in the “Sprycel Case” ruled that in an infringement lawsuit over a patent right with an extended term, the addition or conversion of an additive to a Sprycel tablet does not result in the tablet being substantially identical.
However, we will need to wait for an accumulation of case law regarding the interpretation and thinking behind the technical significance of additives, so it will be important to keep an eye on future case law developments.
https://www.courts.go.jp/assets/hanrei/hanrei-pdf-94302.pdf